Rare Earth Ores Processing Steps
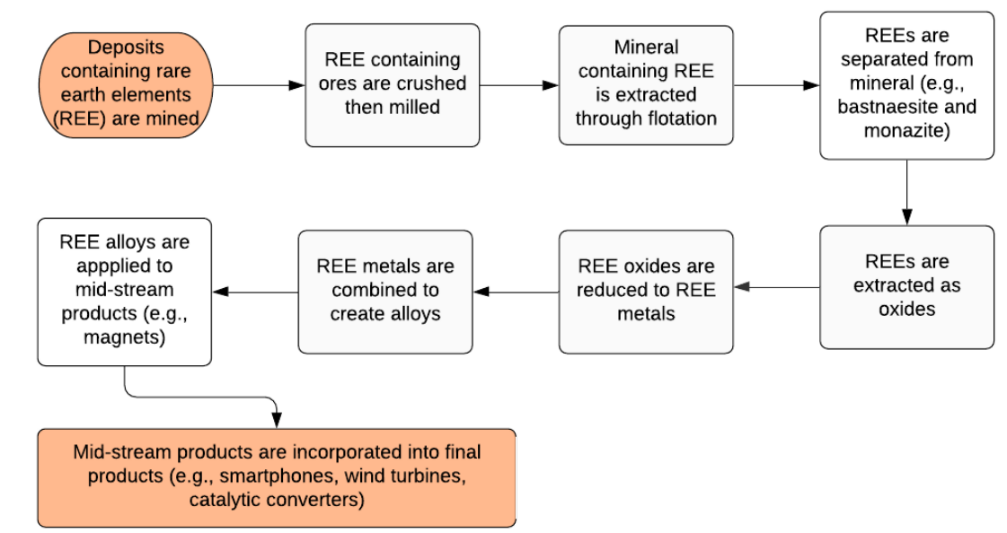
Deposit exploration, mining, beneficiation, chemical treatment, and separation are the five primary processes in the REEs processing process. It is worth noting that before mining, the sector considers economic variables, prospective economic impacts, and environmental concerns. Pure REEs can be found in complicated rocks and recovered through a beneficiation process. To leach the REE concentration, the chemical treatment may take over. Finally, individual elements can be extracted using the hydrometallurgy method, and they are commonly offered to consumers as either pure elements or metal oxides.
Deposit Exploration
The majority of REEs begin with explorations and locating possible RE deposit areas in order to extract valuable minerals that are highly prized around the world. It is worth noting that the relatively larger demand for expensive minerals like gold, copper, RE materials, platinum, and so on has stimulated mining process exploration. The majority of businesses start with sampling and managing comprehensive geochemical analyses. They must engage in greenfield investigation in this regard. Brownfield exploration, on the other hand, is another type of deposit discovery activity, according to some writers, and this strategy carries a smaller risk than greenfield exploration. The descriptions of greenfield and brownfield exploration are summarized in the table below.
|
Category |
Description |
|
Greenfield Exploration |
The focus of greenfield exploration is on forecasting new areas. There are two types: - Grassroots Exploration Project - A geologist discovers a mineral deposit and begins the survey procedure, which includes gravity, magnetism, and radioactivity measurements. - Advanced Exploration Project - A geologist confirms the mineral deposit and proceeds to drill activity. |
|
Brownfield Exploration |
Brownfield exploration activity is different than greenfield activity. The geologists carry on existing mines or proximity operating mines. Brownfield exploration activity is much more interesting because of the low risk and low cost involved in this activity. |
Geologists are currently using the greenfield exploration method to hunt for evidence of metal enrichment by measuring gravity, magnetism, or radioactivity. Detecting radiations, measuring magnetism, fluctuations in the magnetic field, satellite imaging, and geology mapping are all examples of greenfield exploration. The first way is to detect radioactive sources, which are objects that are regarded as acceptable for detecting radioactive elements such as uranium and thorium. The second way is to use magnetometers to measure magnetism, which has been used to detect magnetic minerals. The third way is to look for variations in the magnetic field; this method uses gravimeters to determine mineral density within the earth's crust.
The fourth way is satellite photography, in which geologists take images of certain deposits to identify them, and then the satellite is utilized to generate the element in the maps. Geologic mapping is the fifth approach, which is utilized by geologists to conduct a study on mineral deposits in order to determine their locations and structures. Mechanical supply (machine) and ventilation systems, power and water supply, and other amenities are among the key equipment necessary for mining. Construction normally begins when environmental and safety regulations have been implemented.
Mining
The second step is mining, which follows a regular procedure for producing a variety of minerals and elements. The majority of REEs are mined using one of three methods: surface mining (open pit mining), underground mining, or in-situ leaching.
In RE separation procedures, the open pit approach is often used. This procedure entails blasting and digging the ores, then transporting them to the surface for the next step. Moving on, open-pit mining is often done within 100 meters of the surface, with enormous open-air excavations used to remove the ore prior to processing. This process is less expensive, but it creates big scars on the land surface and is environmentally hazardous. When compared to open-pit mining, underground mining is thought to be more expensive and riskier.
In site leaching rare earth cations are recovered by using chemical exchange processes involving a leaching solution during the seepage process of an ore body, resulting in the extraction of rare earth elements. The pace of rare earth leaching is determined by the degree of chemical exchange and the amount of rare earth mother liquor that seeps into the ore body. Both analytical and anti-absorption methods are included in this ion-adsorption rare earth leaching process.
Beneficiation Process
The beneficiation of RE-bearing minerals is the third phase. The physical separation used in the beneficiation process is used to eliminate unwanted contaminants or increase the concentration of the desired product. The major unit operation in the beneficiation process is the sizing unit (i.e., crushing and grinding), followed by the separation of REO from other minerals using froth floatation (i.e., mixer, froth flotation cell, concentrator), magnetic separation, and gravity separation (i.e., thickening and drying).
RE ores are frequently found in association with barite, fluorite, calcite, silicates, and iron minerals, making it difficult to distinguish between RE minerals and other relationships.
The concentration of REEs has increased as a result of each of the processes. To begin, the REO ores may be crushed and ground in a jaw crusher and ball mill. The crusher is used to reduce the size of the ores to a manageable level. The majority of industries, according to reports, employ crushers and grinders to achieve the appropriate particle size. The ore slurry is subsequently pumped into the next step of beneficiation, which may include froth flotation, magnetic separation, electrostatic separation, gravity concentration, or a combination of these methods.
These techniques are used to extract RE minerals from impurities including copper, zinc, lead, nickel, and other non-ferrous metals. In comparison to other beneficiation procedures, froth flotation is used in industries in a more involved way. This approach separates the selection group of minerals that attach to air bubbles using chemical reagents (i.e., depressants and collectors). To extract important metals and contaminants, the ore-water slurry from the mixer may be sent through a froth floatation cell.
A collector may be required to make RE materials adhere to rising bubbles and float. Air bubbles may rise in tandem with the ore particle-water slurry. In this case, the RE particles may require a minimum diameter of 40-100 m for optimal froth flotation feed. It is worth noting that particles that are not linked to the bubbles have a chance of sinking. Because of the flotation response of their surface qualities, most researchers concentrated on bastnaesite and monazite.
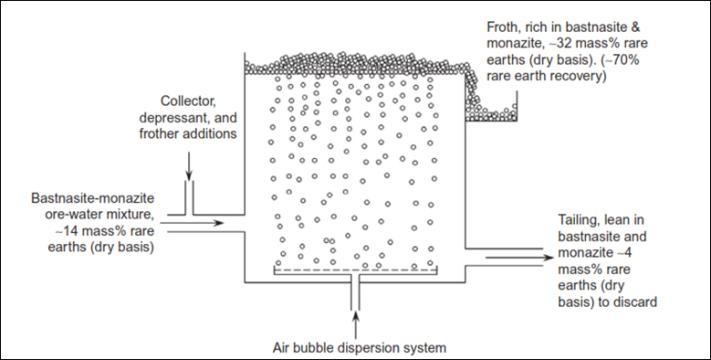
Schematic of froth flotation cell.
Depressants and collectors are two types of chemicals that are commonly employed in froth flotation to separate REEs and other impurity elements. The depressant is a chemical that keeps undesired minerals from floating, whereas the collector is used to attach the minerals to air bubbles and assist them to rise to the surface. Depressants are normally added first, followed by collectors in the ore’s particles.
In comparison to the most commonly used depressants, which include sodium silicate, sodium hexafluorosilicate, lignin sulfonate, or sodium carbonate, traditional chemical collectors for bastnasite minerals are mostly used for hydroxamates, fatty acids, dicarboxylic acids, and organic phosphoric acids. To improve the selectivity of a flotation process, the collector's efficacy is dependent on the particle size, i.e., the viable minerals that were initially floated together. The majority of minerals float at a pH of 7.5-11.5.
Magnetic separation methods are also extensively used in industry to separate highly magnetic gangue from non-ferrous materials such as monazite or xenotime. REEs have a series of electrons that involve a shielded 4f sub-shell, and these electrons have a magnetic charge that does not cancel out, resulting in a material with some magnetism. This combination allows for a high concentration of REE in bastnasite deposits. Xenotime, a paramagnetic mineral, is said to be more magnetically sensitive than monazite.
In fact, RE metals such as neodymium and samarium are commonly used in the production of strong magnets, while rocks containing RE elements are only modestly paramagnetic. The majority of REs are paramagnetic elements, which means they contain fewer magnetic minerals than ferromagnetic minerals (i.e., strongly attracted to a magnet). However, if the ore particle size is less than 100 m, the flotation process is preferred. Gravity separation, according to several authors, is appropriate for minerals with large specific gravities ranging from 4 to 7 and are much less dense, such as silica.
Gravity separation processes, which were placed between the rougher and cleaner flotation circuits to separate both minerals from the iron-bearing and silicate gangue materials, are often used to beneficiate monazite and bastnasite minerals. Gravity separation's downside is that technique is ineffective at separating RE ores with very fine particle sizes, resulting in significant RE losses. Even if the particles are very tiny, gravity separation may be used if there are considerable variances in the specific gravity of the minerals, such as gold from silicate gangue. According to the literature, it is critical to establish a good beneficiation method for physical separation based on the characteristics of the ores. To consider a low-cost and time-saving operation, it is required to first analyze the physical and chemical properties of the ores mineral in order to identify the appropriate method for the beneficiation process.
Dewatering is the final step in the beneficiation process. Water is pumped into a massive concentrator or thickener to handle the ore-water slurry. The particles settle out of the slurry due to gravity, then heat drying is performed using rotary dryers, spray driers, and rotary tray dryers.
Chemical Treatment
Chemical treatment, often known as the cracking process, is required for the fourth phase in RE processing. Chemical therapy can be divided into two types: acid and alkaline. Both techniques are used to raise REOs concentration and remove impurities, with an estimated purity of 90%. Inorganic acids such as sulfuric acid (H2SO4), hydrochloric acid (HCL), and nitric acid (HNO3) were extensively used in acid treatment, whereas NaOH and Na2CO3 were commonly used in alkaline treatment. Because of the phosphate and carbonate–fluoride components of monazite and bastnasite, alkaline treatment is commonly used on them, according to some chemists.
To remove fluoride (HF), sulfur dioxide (SO2), sulfur trioxide (SO3), and silicon tetrafluoride (SiF4), acid-roasting with high temperature and sulfuric acid (H2SO4) was widely used in China. Before being released into the environment, dangerous gases such as hydrofluoric acid (HF), sulfuric acid (H2SO4), and hexafluorosilicic acid (H2SiF6) will be absorbed in the first and second scrubbers. The scrubber is diluted with Na2CO3 solution, which is then utilized to treat the exhaust as it exits.
Other elements found in the leach solution produced by sulphuric acid digestion include uranium (U), thorium (Th), and ferum (Fe). After extracting thorium (Th) and uranium (U), monazite is acid-treated in three different ways: sodium double sulphate precipitation, neutralization by ammonia hydroxide, and neutralization by sodium oxalate. Alkaline treatment is possible in four ways for monazite after minerals salt has removed impurities and radioactive elements, including dissolving by HCL, H2SO, and HNO3. REO was leached from bastnasite by a variety of methods, including calcite REO between 800-900oC, flotation process with calcite by 10% or 30% HCL at 620oC, or dissolution of H2SO4 at 480oC. There are three approaches to leach out REO in xenotime processing: digestion with 93 % H2SO4 at 190-250oC, roasting of Na2CO3 at 900oC, and fusion of NaOH at 400oC. Water is used to leach these techniques, and NH4CL is used to extract them.
Separation Process
The separation procedure is used in the fifth phase to purify individual REOs. Supercritical, biosorption, electro-winning, solvent extraction, and ion exchange are the five most prevalent separation processes. Solvent extraction is the most widely used process in the chemical industry for isolating RE into single elements. Prior to the separation procedure, REE minerals in the form of fluorocarbonates and phosphates must be converted to carbonates or chlorides via exchange or solvent extraction. REO was judged to have a purity of 99 % during this process. Despite this, the same chemical and physical properties of REE make separation challenging, potentially increasing both time and expense of operation.
Chemical precipitation, reduction, bio-sorption, membrane extraction, liquid membranes, solvent extraction, ion exchange, and electro-winning were all recognized as procedures for separating RE from ores. The five most common methods for extracting RE elements are supercritical, bio-sorption, electro-winning, solvent extraction, and ion exchange. Researchers have developed strategies for extracting REE due to the growing number of REE uses.
It is worth noting that numerous scientists are attempting to improve hydrometallurgy technology. The hydrometallurgy method is a metallurgy extraction technology that uses aqueous chemicals to recover valuable metals from ores, as well as a concentration solvent and recycled residual materials. Chemicals' capacity to extract RE mixes from the undesired component by changing the pH and acid/bases used in the hydrometallurgical process is based on their ability to extract RE mixtures from the undesired component by altering the pH and acid/bases used. Individual REs are difficult to distinguish from one another since their physical and chemical properties are often identical. To create high purity single RE solutions or compounds, separation procedures based on ion exchange and solvent approaches have been developed.
Ion Exchange and Solvent Extraction
Because of its capacity to extract and purify RE products, solvent extraction is now widely used in numerous sectors. Several investigations looked at the chemicals and extractants used in the REE extraction process. Solvent extraction is a method of transferring cations or anions from an aqueous phase to an immiscible organic phase using an organic compound (i.e., extractant). REE is started by employing counter-current extraction to separate them according to their groups, such as HREE and LREE. The efficacy of solvent extraction is based on the chemical nature differences between the solvent and aqueous phases.
As a result, variables such as extractant types, primary acidity, ore concentration, and extractant concentration may all play a role in effective RE separation. Separation of REEs using multiple-unit mixer settlers is a frequent way to the required individual element of RE. The solvent extractant, which is an organic phase, and the RE leached, which is an aqueous phase, are combined in the mixer, where the particles settle out and are separated by gravity. Notably, in some cases in the business, mixers with centrifugal force have been constructed to mix both organic and organic phases. The settling time, according to many writers, limits the efficiency of the settler. The most practicable way for separating the RE was determined to be the ion exchange procedure.
Although ion exchange is another method of separating RE that has been used to achieve 99.99% purity in REO, it is not considered cost-effective. To get high-quality RE products for electrical or analytical applications, most ion exchange processes are used. Cation exchangers, anion exchangers, and solvating extractants, according to some writers, are the three major categories of extractants. The solvating extractant is the most widely utilized among these extractants in the business. According to some studies, di-2-ethylhexyl-phosphoric acid (D2EHPA or P204) and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (HEH/EHP or P507) are common extractants used in the business.
Supercritical Fluid
The supercritical fluid (SCF) method is a carbon dioxide-based process for extracting RE components (CO2). This approach uses carbon dioxide and involves raising and compressing the temperature until it reaches a critical temperature and pressure. The majority of studies are interested in REEs extracted using the SCF approach, in which REEs extraction should be increased by increasing the mass transfer between CO2 and REEs. After the extraction, the solute is rapidly and completely removed from the solvent by gasification of carbon dioxide at air pressure. In the extraction of lanthanum and europium, an 87 percent ratio was attained.
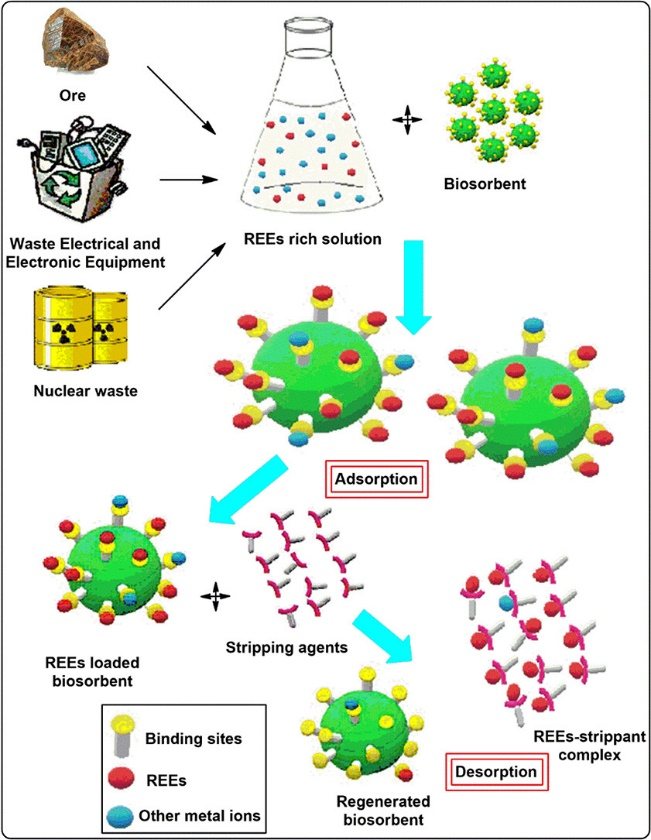
Bio-sorption
The biosorption method is a new biological method for sequestering metallic cations from diluted aqueous solutions that have gotten a lot of attention from scientists.
According to a lot of experts, the most empirical advantage of adopting biosorption is its cost-effective factor, which is linked to the use of natural biomass to separate the RE elements.
It is important to remember that sorption is dependent on the type of microorganism or bio-sorbent used, as well as the experimental settings. The biosorption process is influenced by a number of elements, including pH ranges of 4 to 7, bio-sorbent dosages of 15-200 mg/L, the temperature of 25-60oC, starting metal concentrations of 15-300 mg/L, and contact time of 300-480 minutes.